12 Nov, 2024

12 Nov, 2024
Chimeric antigen receptor (CAR) T-cell therapy is an advanced therapy for treating various hematological malignancies and solid organ tumors. It uses the immune system to make the immune cells more effective against the cancer cells. It is done by genetically modifying the T-cells to express the receptors specific to target cancer cells.
Some of the common types and symptoms of cancer in men are:
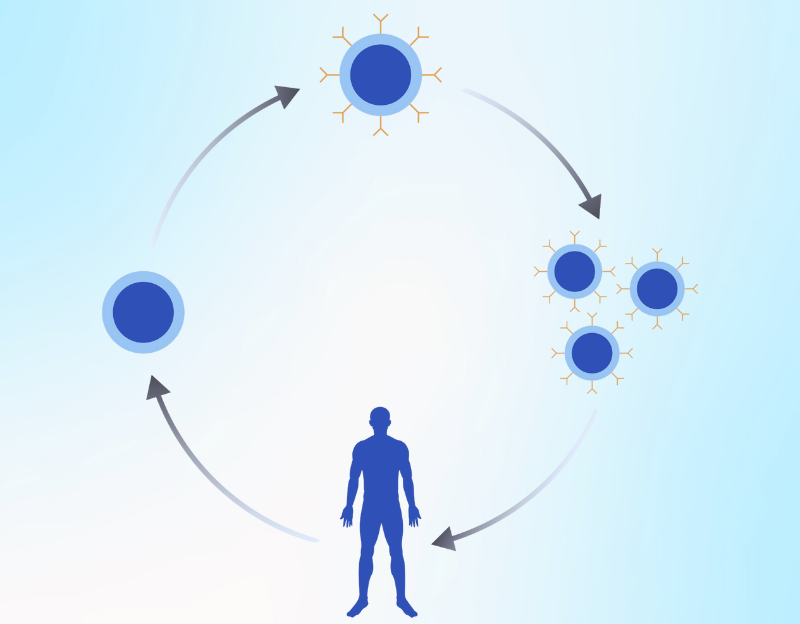
Foreign substances have proteins on the surface of their cells. These proteins are known as antigens. The T-cells of the immune system have receptors on their surface that bind to these antigens. The binding of the receptor to the antigen activates other immune system cells and destroys foreign substances. It is to be noted that the interaction between the receptor and the antigen is based on the lock and key model. Unless there is a right receptor on the T-cells, the immune system does not recognize foreign substances as an antigen. Cancer cells also have antigens on their surface. However, in the absence of any receptor on the T-cells that bind the antigen, the cancer cells evade the immune system's attack and proliferate.
During the CAR T-cell therapy, the T-cells of the patients are taken from the blood, and a gene for a receptor is added. The receptor is known as the chimeric antigen receptor (CAR). The modified T-cells are then infused back into the patient. The presence of the chimeric antigen receptor allows the T-cell to bind the antigen on the surface of cancer cells.
A procedure known as leukapheresis is used for collecting the T-cells from the patient's blood. In this process, the patient lies on the bed, and two IV lines are attached. The blood is drawn from the vessels through one IV line and guided into the machine, which separates the white blood cells. The blood is sent back into the body through the second IV line. Some patients may have low calcium after the procedure, which can be managed through IV or oral calcium supplementation.
Once the white blood cells are obtained, the T-cells are separated and sent to the molecular laboratory. A specific chimeric antigen receptor is added to T-cells to obtain CAR T-cells. These CAR T-cells are grown in the laboratory for several weeks to obtain the appropriate number required for therapy.
The procedure of isolation and reinfusion of lymphocytes is termed as adoptive cell transfer. Before infusing the CAR T-cells back into the patient's circulation, they may be given chemotherapy to lower the count of other immune cells. It will improve the working of CAR T-cells against cancer cells.
Although CAR T-cell therapy is a novel and effective method for managing various types of cancer, it has certain limitations. Some of the limitations include:
It has been found that malignant cells in patients treated with Chimeric antigen receptor T-cell therapy have partially or completely lost the target antigen expression in many patients. This mechanism is known as antigen escape and may result in CAR T-cell therapy failure. This trend has been reported in patients suffering from acute lymphoblastic leukemia, glioblastoma, and multiple myeloma.
The solid tumor antigens are often expressed on the normal healthy cells at different levels, resulting in “on-target off-tumor” toxicity that may lead to CAR T-cell therapy failure. It is thus important to carefully select the target antigen to avoid toxicity from the CAR T-cell therapy.
The penetration and mobility of CAR T-cell therapy are limited by physical barriers, such as tumor stroma and immunosuppressive microenvironment of the tumor, resulting in reduced efficacy and CAR T-cell therapy failure. The mechanism is more common when managing solid organ malignancies than hematological malignancies. These limitations can be overcome by delivering the therapy through other routes of administration in addition to systemic administration.
Although CAR T-cell therapy is highly effective in some cancer types, it does not achieve the status of first-line therapy due to high incidences of toxicities, which may sometimes be fatal. Some symptoms of CAR T-cell therapy toxicity include splenomegaly, renal failure, and pulmonary edema.
In the tumor microenvironment, there is an infiltration of various immunosuppressive cells. These cells include regulatory T-cells, tumor-associated macrophages, and myeloid-derived suppressor cells. There is an increased synthesis of chemokines, cytokines, and growth factors. CAR T-cell therapy may have no or weak response due to short-term persistence and poor expansion of T-cells in such an immunosuppressive environment leading to CAR T-cell therapy failure.
There have been significant advancements in CAR T-cell therapy during the last decade. Drug regulatory authorities have approved several drugs; however, acceptance is relatively slower due to several barriers. However, recent advancements in the immunotherapy domain have the potential to increase its application. There are over 1400 drugs in the pipeline and over 800 trials going on in CAR T-cell therapy.
Almost all cases of multiple myeloma relapse, possibly due to drug resistance. However, advanced therapies, such as CAR T-cell therapy, improve the mean survival period in these patients. There are at least two FDA-approved CAR T-cell therapies for patients with multiple myeloma. The antigen targeted by the CAR T-cell therapy in multiple myeloma is the B-cell maturation antigen (BCMA). Several drug products related to CAR T-cell therapy for treating multiple myeloma are under investigation for safety and efficacy.
T-cell cancer can be classified into peripheral, T-cell lymphomas, and T-cell leukemia. Unlike the CAR T-cell therapy targeted for B-cell malignancies, the CAR T-cell therapy for T-cell cancer does not target CD 19 and BCMA as these are not expressed in T-cells. Further, CAR T-cell therapy targeting T-cell cancers also kills the CAR T-cells and normal T-cells due to mutual T-cell antigens. There are ongoing clinical trials related to antigens targeted for T-cell cancers, including CD4, CD5, CD7, CD30, and CD37.
Unlike in hematological cancers, CAR T-cell therapy has not shown relatively similar efficacy in managing solid tumors, probably due to immunosuppressive tumor microenvironments and heterogeneous tumor antigen expression. The advanced strategies to overcome these barriers to make the CAR T-cell effective in solid tumors include delivering CAR T-cells with vaccines, integrating chemokine receptors into the CAR T-cell, genetic modification of CAR T-cells for IL-8 receptor expression, using glycoengineering, blocking protein kinase A, and conducting regional hyperthermia (photothermal therapy).
Several approaches have been used to develop the next-generation CAR T-cells to effectively manage hematological cancers and solid tumors. Some of these approaches include co-administering CAR T-cells for different target antigens to avoid antigen escape, developing CAR T-cells for antigen-unrestricted cytokine-initiated killing (TRUCK) that secrete cytokines, combining CAR T-cells with hematopoietic stem cell transplantation to prevent target / off-tumor toxicities, and delivering engineered viruses to modulate tumor microenvironment.
CAR T-cell procedure has been shown to be effective in managing chemotherapy-refractory B-cell malignancies and is approved for B-cell malignancies and multiple myeloma. However, the challenges in treating these diseases include the absence of tumor-specific antigens. Recently, a new antigen target, CD84, has been studied and found to be effective, both in-vivo and in-vitro, for treating relapsed and refractory acute myeloid leukemia.
Several studies have been performed to determine the safety and efficacy of CAR T-cell therapy in children and young people. However, only one therapy, i.e., tisagenlecleucel, is an FDA approved CAR T-cell therapy for managing B-cell ALL that is refractory or in a second relapse in patients up to 25 years of age.
Several CAR T-cell therapies have been approved in adults for the management of a variety of cancers. These include idecabtagene vicleucel, lisocabtagene maraleucel, ciltacabtagene autoleucel, tisagenlecleucel, brexucabtagene autoleucel, and axicabtagene ciloleucel.
Like other cancer therapies, there are certain CAR T-cell therapy side effects. Some of these include:
Multiplication of CAR T-cells releases significant cytokines in the blood, leading to elevated immune reactions and several CAR T-cell therapy side effects:
The symptoms of CRS include trouble breathing, headache, tiredness and fatigue, lightheadedness, severe diarrhea, nausea, vomiting, rapid heartbeat, joint and muscle pain, fever and chills, and low blood pressure.
CAR T-cell therapy side effects are also presented in the nervous system and causes seizures, headaches, altered consciousness, loss of balance, tremors, speaking and comprehending problems, and confusion.
Other CAR T-cell therapy side effects include a compromised immune system, low sodium, potassium, and phosphorus serum levels, allergic reaction during administration, and reduced blood cell count (resulting in increased infection risk, bleeding, and fatigue).
CAR T-cell therapy targeting CD19 is highly effective in managing relapsed or refractory B-cell lymphomas. The durability of the response, along with the response rate, was excellent, and USFDA approved the therapy for several lymphoma subtypes.
Brexucabtagene autoleucel has been approved for treating adults with refractory or B-cell acute lymphoblastic leukemia.
Some of the FDA approved CAR T-cell therapy includes:
The target antigen for this drug is BCMA, and it is approved in adults with relapsed or refractory multiple myeloma.
The target antigen for this drug is CD19, and it is approved in adults with relapsed and refractory B-cell non-Hodgkin's lymphoma.
The target antigen for this drug is BCMA, and it is approved in adults with relapsed or refractory multiple myeloma.
The target antigen for this drug is CD19, and it is approved in young adults and children with refractory or relapsed B-cell acute lymphoblastic leukemia and in adults with relapsed and refractory B-cell non-Hodgkin's lymphoma.
The target antigen for this drug is CD19, and it is approved in adults with relapsed or refractory Mantle cell lymphoma and refractory or relapsed B-cell acute lymphoblastic leukemia.
The target antigen for this drug is CD19, and it is approved in adults with relapsed or refractory follicular lymphoma and adults with relapsed and refractory B-cell non-Hodgkin's lymphoma.
Several factors affect the CAR T-cell therapy cost. These are:
Cancer patients undergo several tests, such as comprehensive physical examination, blood tests, liver function tests, and kidney function tests, to determine if the patient can tolerate CAR T-cell therapy. Further, the oncologists also perform tests on the cancer cells to determine if CAR T-cell therapy is effective in a particular patient. These tests are added to the CAR T-cell therapy cost.
In the case of solid tumors, CAR T-cell therapy usually accompanies surgery to remove the tumor. The type of surgery performed also affects the CAR T-cell therapy cost.
The CAR T-cell therapy cost is also increased due to chemotherapy or radiotherapy sessions usually done before the CAR T-cell therapy in India.
HCG is one of the most advanced cancer care centers, with all the treatment options required to manage simple to advanced cancers. The center has advanced diagnostic techniques that allow accurate and early cancer diagnosis. The hospital has the facility to deliver CAR T-cell therapy in India. Further, the genetic laboratory at the center also helps the oncologists to determine if CAR T-cell therapy is effective in a particular patient. The cancer specialists at the HCG are extensively experienced in delivering advanced therapies, such as CAR T-cell therapy.
Chimeric antigen receptor T-cell therapy revolutionized cancer treatment, especially refractory and relapsed hematological cancers. Several studies have been performed to prove the safety and efficacy of this therapy, and several drugs have been approved for the treatment of cancers, such as B-cell acute lymphoblastic leukemia, follicular lymphoma, Mantle cell lymphoma, and multiple myeloma. However, because of the side effects, some of which are serious, patients undergoing CAR T-cell procedure are under continuous monitoring. Various drugs targeting specific antigens in cancer cells are under clinical trials.
The CAR cells remain in the system for months and years in many patients. However, the treatment can be safely repeated if the CAR cells are rapidly lost from the system.
The time for infusing CAR cells in cancer patients ranges from 15 minutes to one hour.
The success rate of CAR T technology depends upon several factors, such as the type of cancer and the overall health of the patients. For instance, studies have shown that the complete response rate with CAR T-cell was 71–81% in patients with B-cell acute lymphoblastic leukemia.
Although several Chimeric antigen receptor T-cell therapy drugs have been approved by USFDA in adults, only one, i.e., tisagenlecleucel, has been approved in patients less than 25 years of age.
Some alternatives to the CAR T-cell procedure include monoclonal antibodies and stem cell or bone marrow transplantation.
The patients undergoing Chimeric antigen receptor T-cell therapy may recover within 2 to 3 months.
